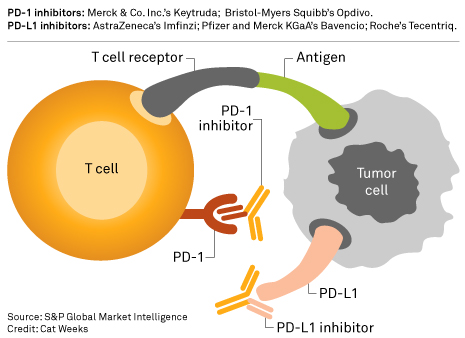
US FDA panel backs Merck & Co.'s Keytruda to treat type of bladder cancer | S&P Global Market Intelligence

Merck's PD-1 Drug Outperforms Ipilimumab for Treatment of Advanced Melanoma - Cancer Research Institute
FDA Approves Mercks KEYTRUDA® for Patients with Recurrent or Metastatic Head and Neck Cancer | World Pharma Today
FDA Approves Merck's KEYTRUDA for the Adjuvant Treatment of Patients with Melanoma with Involvement of Lymph Node | World Pharma Today

FDA Accepts Application for Merck's KEYTRUDA® (pembrolizumab) Plus Chemotherapy as Treatment for Advanced or Unresectable Biliary Tract Cancer
KEYTRUDA® (pembrolizumab) Plus Padcev® (enfortumab vedotin-ejfv) Reduced Risk of Death by More Than Half Versus Chemotherapy in Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer | PharmiWeb.Jobs United States

Seagen, Astellas and Merck Announce FDA Acceptance of sBLAs for PADCEV® (enfortumab vedotin-ejfv) with KEYTRUDA® (pembrolizumab) for the First-Line Treatment of Certain Patients With Locally Advanced or Metastatic Urothelial Cancer
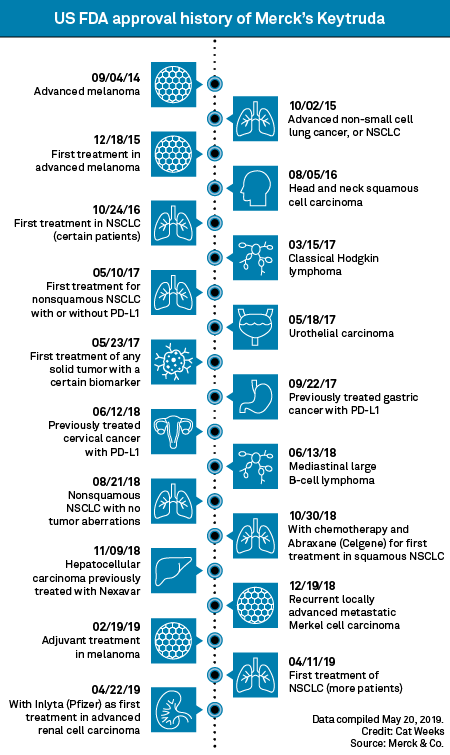
US FDA approves Merck & Co.'s Keytruda in 2 new head, neck cancer uses | S&P Global Market Intelligence

Moderna on X: "We announced today that mRNA-4157/V940, an investigational personalized #mRNA #cancer vaccine, in combination with KEYTRUDA, @Merck's anti-PD-1 therapy, has been granted Breakthrough Therapy Designation by the U.S. FDA for

Keytruda Approved by FDA for Further Potential Treatments of Mesothelioma | Mesothelioma Help Cancer Organization